Healthcare data breaches are growing fast, and the numbers proves it.
Between 2009 and 2024, there were 6,759 healthcare data breaches of 500 or more records reported to the Office for Civil Rights (OCR). Those breaches exposed or impermissibly disclosed the protected health information of 846,962,011 individuals, more than 2.6 times the population of the United States.
In 2023, an average of 1.99 healthcare data breaches were reported each day, compromising around 364,571 records daily. The trend continued into 2024, with 276,775,457 medical records exposed or stolen, an average of 758,288 every single day.
(Source: HIPAA Journal, Oct 2025)
Every one of these records contained sensitive PHI that should have been protected or redacted. For research teams handling medical data, even a single unmasked identifier can trigger a compliance issue or breach of trust.
Manual redaction is slow and prone to human error. AI-powered PHI redaction now makes it possible to automatically scan and remove identifiers from medical records in minutes, ensuring faster workflows and full HIPAA compliance.
This guide explains how to redact PHI in medical records automatically for clinical research.
What Is PHI and Why Does It Matter in Clinical Research
Protected Health Information (PHI) includes any data that can identify a patient, such as names, addresses, contact numbers, and medical record numbers. Under the Health Insurance Portability and Accountability Act (HIPAA), researchers must remove or obscure these identifiers before sharing medical records, videos, or scanned documents for study purposes.
In addition to HIPAA, several global and national data privacy laws like the Freedom of Information Act (FOIA), General Data Protection Regulation (GDPR), and the HITECH Act influence how healthcare institutions handle patient information. These frameworks collectively emphasize safeguarding sensitive medical data, limiting disclosure, and ensuring accountability in data sharing. For clinical researchers, understanding these overlapping compliance requirements is crucial to maintain both legal and ethical standards when working with patient records.
Failure to do so can lead to:
- Compliance violations and penalties
- Revocation of IRB approvals
- Breach of patient confidentiality and data trust
Challenges of Manual PHI Redaction
Manual redaction challenges are not limited to paper-based files; they also extend to electronic health records (EHRs) and release-of-information (ROI) processes. In many healthcare organizations, managing PHI across these digital systems introduces added complexity, as sensitive data can be stored in structured databases, scanned documents, and patient portals. Ensuring proper redaction across all these formats is a critical challenge for compliance teams.
Redacting PHI by hand might seem simple, but in large-scale research operations, it becomes a bottleneck.
Here’s why manual redaction is no longer sustainable:
- Time-Consuming: Redacting 500–5000 medical records per month can take hours of staff time.
- Human Error: Even trained staff can miss identifiers hidden in scanned text or handwritten notes.
- Inconsistency: Different reviewers apply different standards, risking compliance gaps.
- Scalability Issues: As research data grows, manual workflows can’t keep up.
For example, a research team conducting multicenter trials may need to blind hundreds of documents and even redact accompanying video or audio interviews of trial subjects (patients) weekly, something nearly impossible to manage manually without errors.
How AI Automates PHI Redaction in Medical Records
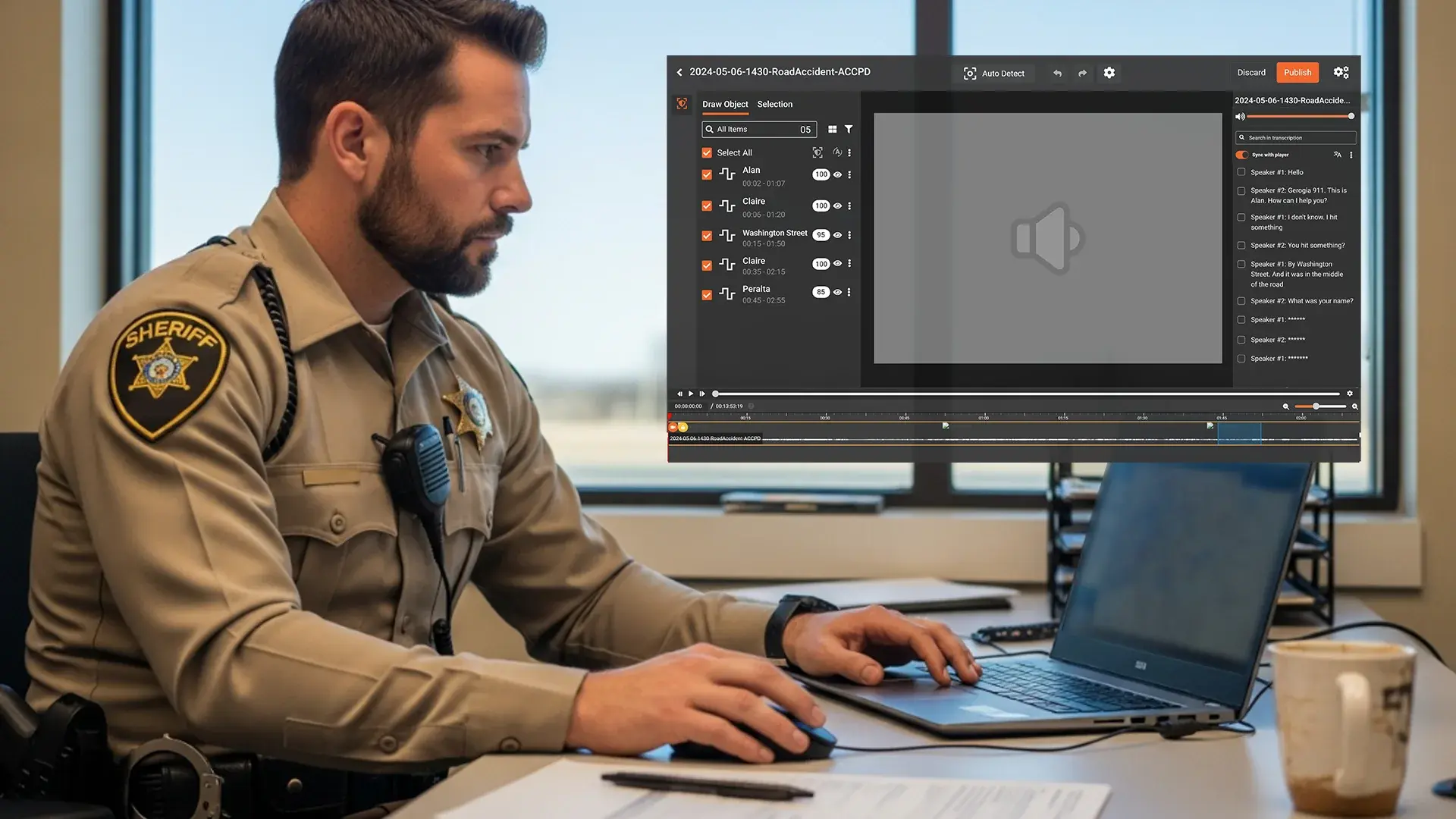
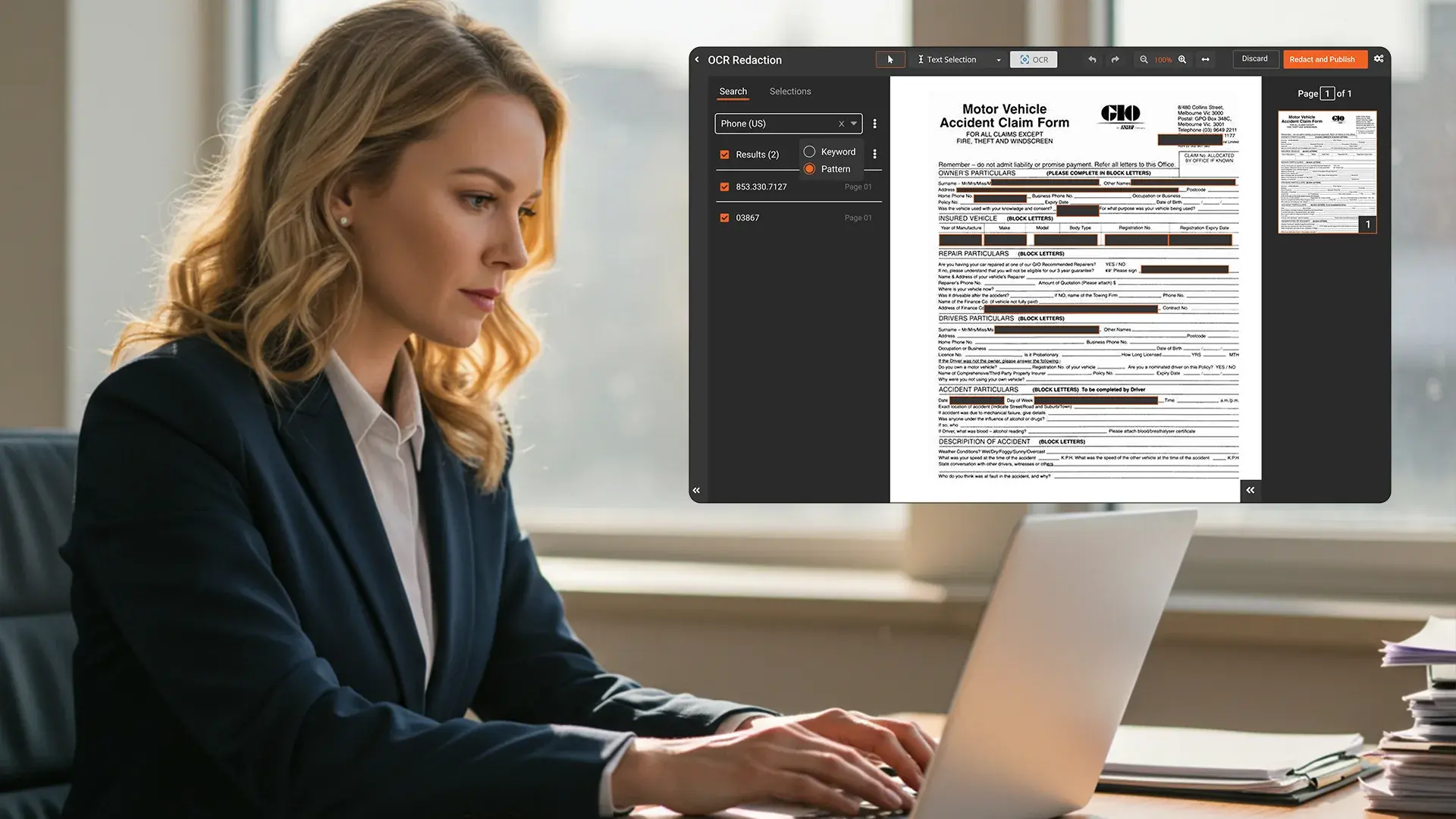
Modern AI-based redaction tools can detect and redact PHI across multiple file types, video, audio, images, documents, as well as scanned PDFs, using machine learning and optical character recognition (OCR).
Here’s how it works step-by-step:
1. Upload Medical Records
Upload PDFs, scanned forms, or audio, video consultations containing PHI.
2. Automatic Detection
The AI scans for identifiers like patient names, contact details, medical record numbers, and dates of birth, using pre-trained healthcare models.
3. Automatic Redaction or Blurring
PHI is automatically removed, masked, or blurred depending on file type and settings.
4. Audit Trail & Review
Each redaction is logged and reviewable, ensuring transparency for audits or IRB compliance reviews.
5. Export Clean Data
Researchers can download redacted versions immediately, ready for sharing or storage.
Benefits of Automatic PHI Redaction
There are many benefits to automated PHI redaction, including the ability to reduce audit risks and maintain a complete audit trail for compliance checks. Automated tools log every redaction action and ensure traceability during HIPAA or IRB reviews, giving healthcare organizations greater confidence in their data handling processes.
For Clinical Research Teams:
- Save Time: Reduce hours of manual work into minutes through batch redaction.
- Ensure Accuracy: AI models catch details humans may overlook.
- Stay HIPAA-Compliant: Built-in compliance with HIPAA, GDPR, and research ethics standards.
- Enable Collaboration: Share de-identified data safely with external collaborators or research partners.
- Support Multiple Formats: Handle text, scanned forms, and even video files from telehealth sessions.
Key Takeaways
- Manual PHI redaction is slow and risky.
- AI-powered tools can automatically redact PHI across formats like PDFs, videos, and images.
- Automated workflows reduce errors and maintain HIPAA and IRB compliance.
- Clinical research teams can securely share de-identified data while focusing on their studies.
To explore how AI-based redaction software can help streamline compliance, you can explore solutions like VIDIZMO Redactor, built to automate and simplify PHI redaction workflows for healthcare and research organizations.
Conclusion
As data-driven healthcare research continues to expand, compliance with HIPAA and patient confidentiality remains non-negotiable.
Automating PHI redaction ensures your research team maintains accuracy, security, and compliance without losing valuable time to manual processes.
Start simplifying your workflows today with a reliable AI-powered redaction tool designed to support healthcare, legal, and government compliance, such as VIDIZMO Redactor.
Get a Free Trial - No Credit Card Needed




No Comments Yet
Let us know what you think